About us
Neurovascular diseases (NVDs) are the leading cause of long-term disability, a major cause of dementia, and the second most common cause of death worldwide. Despite their enormous societal impact, disease-specific therapeutic options remain limited. Recent advances in neurovascular research have revealed an unexpected biological complexity underlying these disorders, pointing to novel mechanisms and therapeutic opportunities that are only beginning to be understood.
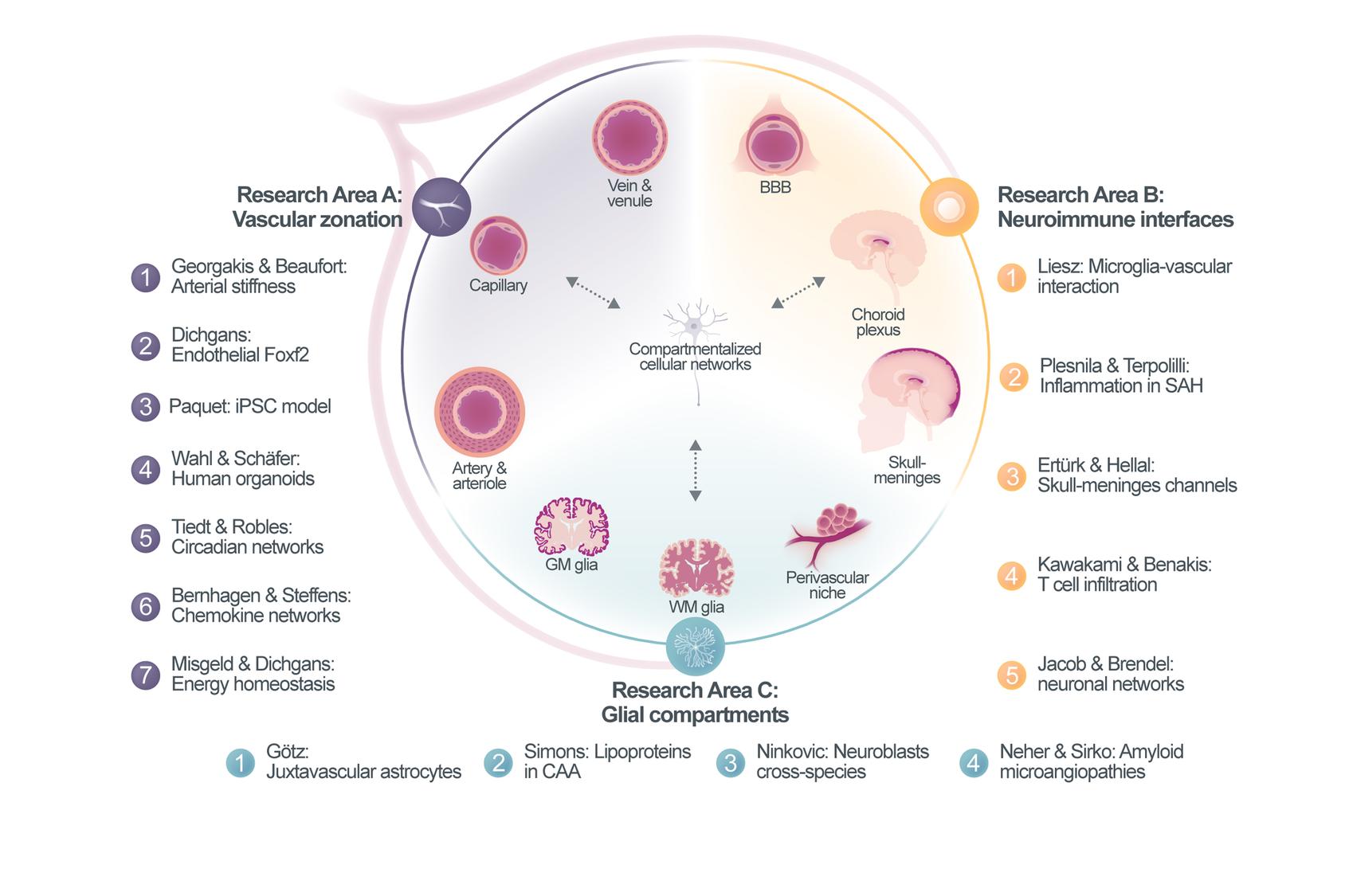
A central insight emerging from single-cell and spatial omics as well as advanced imaging technologies is the remarkable cellular diversity and compartmentalization of the brain. Distinct anatomical and functional compartments host highly specialized cell populations, including zonation-dependent endothelial and mural cells along the vasculature, unique immune interfaces such as the choroid plexus and meningeal spaces, and glial cells that adopt compartment-specific functional states, for example within the perivascular niche. These compartmentalized cellular environments are increasingly recognized as critical determinants of neurovascular health and disease.
The Collaborative Research Center CRC 1744 is built around the concept that compartmentalized cellular networks (CCNs)—dynamic and highly coordinated interactions between vascular, immune, and glial cells—govern the initiation, progression, and outcome of neurovascular diseases. These cellular networks support neuronal function under physiological conditions but can also drive disease processes when dysregulated, thereby representing promising targets for therapeutic intervention.
Our research program aims to elucidate how interactions between compartment-specific cell types and their context-dependent responses shape disease trajectories across a spectrum of neurovascular disorders. We focus on both chronic and acute conditions that directly affect the brain vasculature, including cerebral small vessel disease, cerebrovascular amyloidosis, ischemic stroke, and intracerebral and subarachnoid hemorrhage. By studying these diseases in parallel, we seek to identify shared and disease-specific mechanisms of vascular dysfunction, immune activation, glial responses, and neuronal impairment.
CRC 1744 brings together a multidisciplinary consortium of experts in neurovascular biology, immunology, glial and stem cell biology, genetics, imaging, and data science. Our methodological approach integrates single-cell and spatial transcriptomics, state-of-the-art imaging, and targeted genetic manipulations across harmonized experimental models. In addition to animal models, we employ advanced human iPSC-based systems of the neurovascular unit, transplanted human brain organoids, and exploratory in-vivo multicellular recordings in patients. Shared platforms and standardized model systems across projects enable comparative analyses and foster strong scientific synergies within the consortium.
Our long-term goal is to translate mechanistic insights from experimental and human model systems into clinically relevant concepts and therapeutic strategies that target disease-driving processes within specific neurovascular compartments. Addressing the complexity of neurovascular diseases requires precisely this collaborative framework: a coordinated, interdisciplinary research structure that enables integration of expertise, technologies, and data to advance our understanding of neurovascular biology and disease.