Research Area C: Glial Compartments
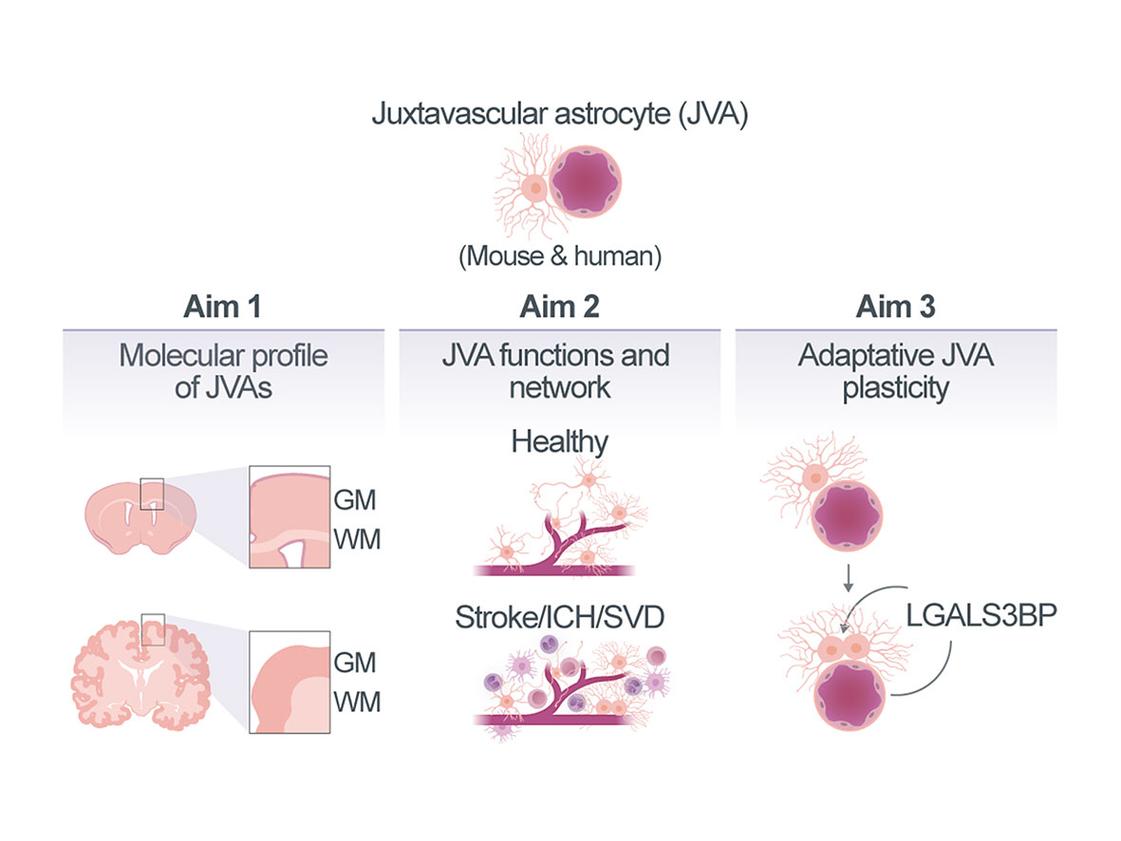
Function of astrocytes at the juxtavascular interface in health and disease
Scarring and inflammation play a key role in neurovascular disease (NVD). We have previously identified that specifically astrocytes within the neurovascular compartment, namely the juxtavascular astrocytes (JVAs), which are located with their soma closely attached to the blood vessel (BV), play a beneficial role in scar formation by restraining the invasion of monocytes. This is correlated to their proliferation, as increasing their proliferative activity reduced monocyte invasion and scar formation. Excitingly, these proliferating astrocytes were also detected in human brain pathology, including NVD, specifically in disease conditions with intracerebral haemorrhage (ICH), as also shown in mice1. In pathology we found Galectin3 (LGALS3, a β-galactoside-binding lectin) and its binding protein LGALS3BP to be important regulators for astrocyte proliferation in the Grey Matter (GM). However, virtually nothing is known about the specific cellular JVA interactions in this niche in intact tissue, nor during aging or in pathology. Excitingly, we have recently identified a JVA population in the White Matter (WM) that is highly proliferative in the intact brain. Therefore, we aim here to i) identify the molecular signatures and cellular interactions of JVAs in the compartments of cerebral GM and WM in the intact, and aging murine and human brain, with particular focus on vessel zonation, to determine whether these interactions differ at interfaces of distinct vessel types and how they are affected in aging; ii) investigate the changes in gene expression, cellular interactions and function, such as proliferation, in JVA and the neurovascular compartment upon (a) transient opening of the Blood-Brain-Barrier (BBB) by focused ultrasound, (b) Small Vessel Disease (SVD) in the genetic model of endothelial cell (EC)-specific Foxf2 deletion, and (c) full blown ICH modelled by collagenase injection; and iii) determine the molecular mechanism by which LGALS3-LGALS3BP signaling promotes JVA proliferation in human astrocyte in vitro models, including our newly establish adult human brain slice culture system, and determine the functional outcome of manipulating this pathway in ICH murine in vivo models. Ideally, we also aim to manipulate novel candidates emerging from our better understanding of the JVA interactions and signaling pathways in the neurovascular compartment.
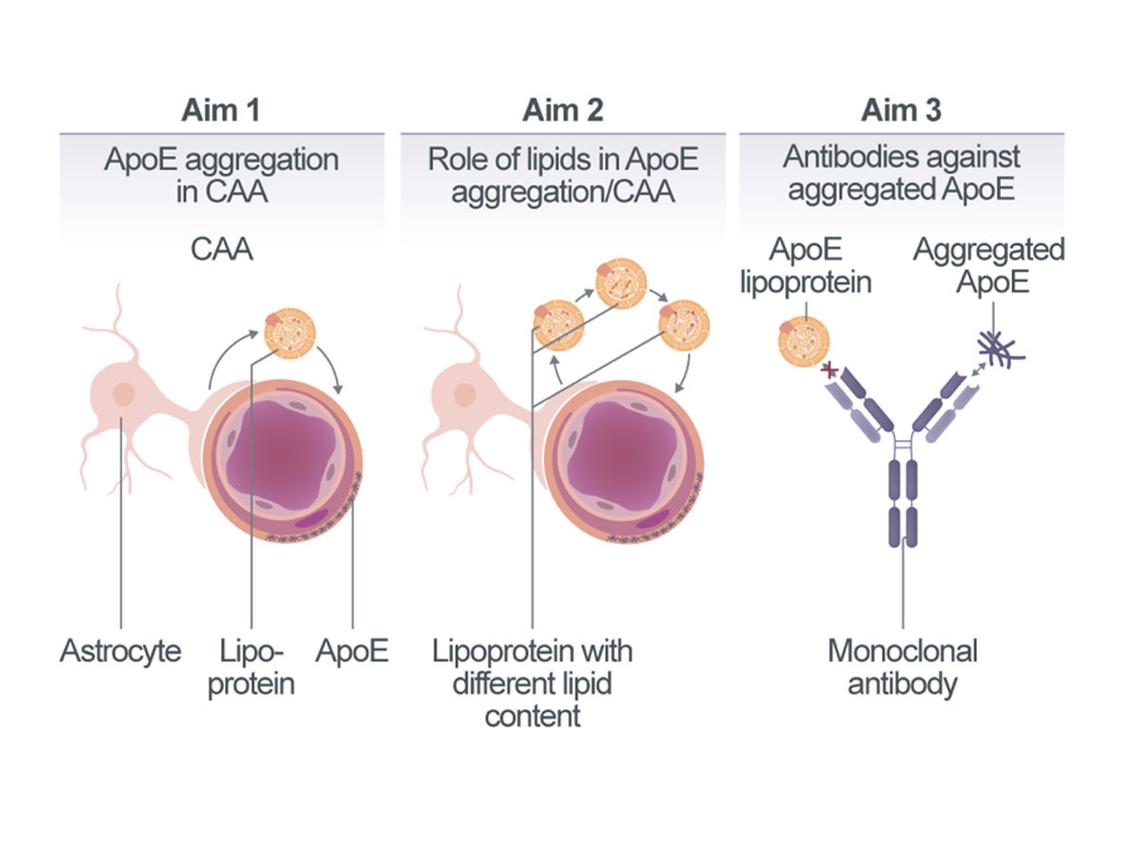
Role of lipoproteins in cerebral amyloid angiopathy
APOE4 is the strongest genetic risk factor for sporadic cerebral amyloid angiopathy (CAA), contributing to vascular amyloid accumulation, vessel fragility, and increased risk of dementia. While APOE4 is hypothesized to impair Aβ clearance across the blood-brain barrier and disrupt perivascular drainage, the precise mechanisms by which it promotes CAA remain poorly understood. Our previous findings suggest that APOE aggregation itself may drive β-amyloidosis. Building on this, we propose that astrocyte-secreted APOE lipoparticles become trapped within cerebral vessels, where they aggregate and serve as nucleation sites for vascular amyloid deposition. We hypothesize that the enhanced aggregation propensity of APOE4 underlies its pathogenic role in CAA. To test this, we will investigate co-factors that regulate APOE aggregation, focusing particularly on APOE-lipid interactions. We aim to map the cellular network that controls lipoprotein secretion and aggregation in the context of CAA. Specifically, we will characterize the spatial and temporal dynamics of APOE deposition along the arterial-to-venous axis, identifying when and where aggregates form, whether they originate from glial or vascular cells, and whether they arise intra- or extracellularly. We will also assess whether APOE aggregates actively seed vascular amyloid and determine the specific contribution of the APOE4 isoform. Using lipid dyes and mass spectrometry-based lipidomics, we will evaluate whether lipids co-deposit with APOE in cerebral vessels. To further examine the role of lipids in CAA pathogenesis, we will manipulate the lipid composition of APOE lipoparticles via mouse genetics and assess the impact on disease progression. Finally, we will explore aggregated APOE as a therapeutic target by developing antibodies against aggregated APOE and testing their efficacy in preventing or reducing CAA.
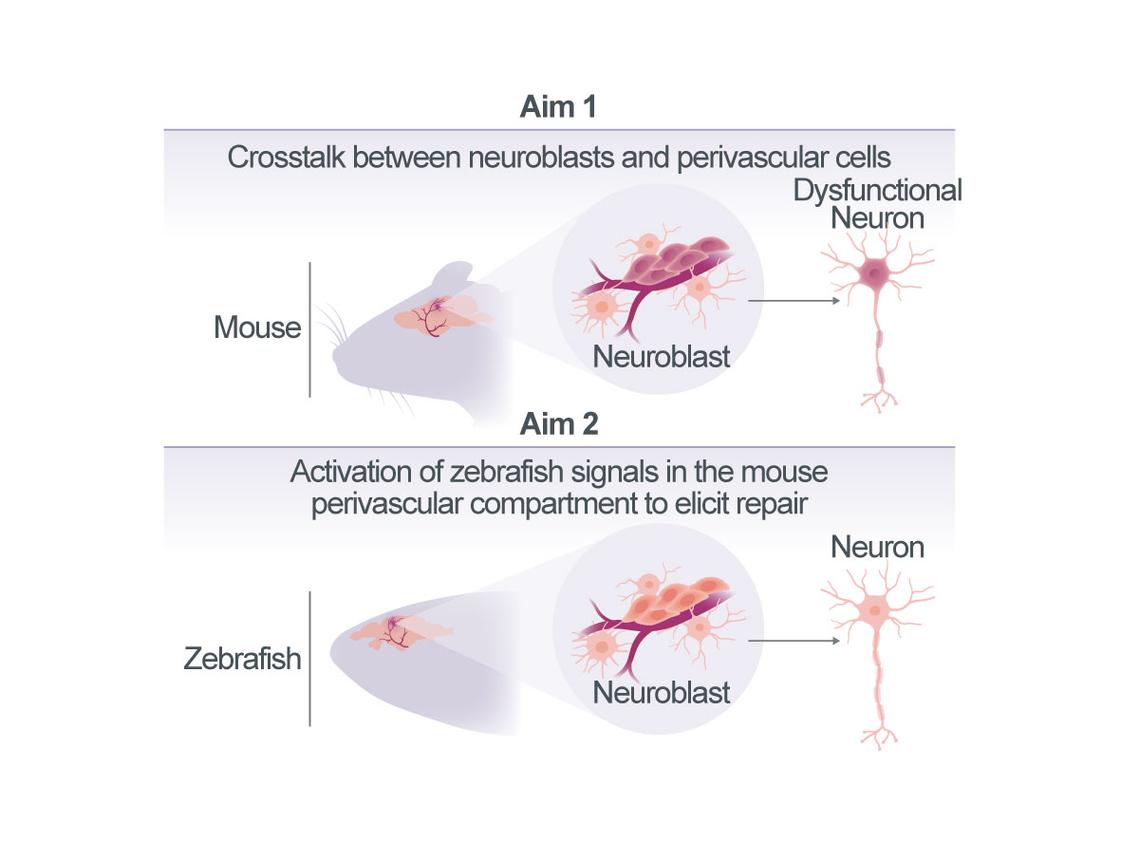
Promoting neuroblast maturation to improve recovery post-stroke
Stroke is associated with the accumulation of neuronal progenitors – referred to as induced neuroblasts (iNBs) – within the perivascular compartment of infarcted tissue. This accumulation appears to involve interactions between iNBs and surrounding endothelial cells and astrocytes. Notably, the recruitment of iNBs to the perivascular niche is a stroke-specific response and has not been observed in other central nervous system pathologies, such as traumatic brain injury (TBI) or chronic neuroinflammation. However, despite their accumulation, iNBs fail to differentiate into functional neurons under these conditions and therefore do not contribute to regeneration after stroke. This project aims to characterize the iNB-containing compartment that forms in response to stroke and to identify the signaling mechanisms that may be impairing neuronal maturation in mammalian species. To investigate this, we will conduct comparative, cross-species analyses of the cellular response to stroke in both mice (Mus musculus) and zebrafish (Danio rerio).
In Aim 1, we will define the precise cellular composition of the iNB-containing compartment in peri-infarct area of the post-stroke brain tissue from both mice and zebrafish. In Aim 2, we will perform a single-cell level comparison of the cellular responses and intercellular signaling within the iNB niches of both species. Because zebrafish are capable of complete brain regeneration following injury, signaling pathways uniquely active in zebrafish represent promising candidates for promoting neuronal regeneration after stroke in mammals. Once these candidate pathways are identified, we will assess their functionality in the mouse cerebral cortex and determine their specific roles in promoting neuronal differentiation within the post-stroke perivascular compartment. A detailed understanding of the cellular composition and signaling dynamics of the iNB-containing perivascular niche will provide a critical foundation for the development of therapeutic strategies aimed at inducing neuronal replacment in patients recovering from stroke.
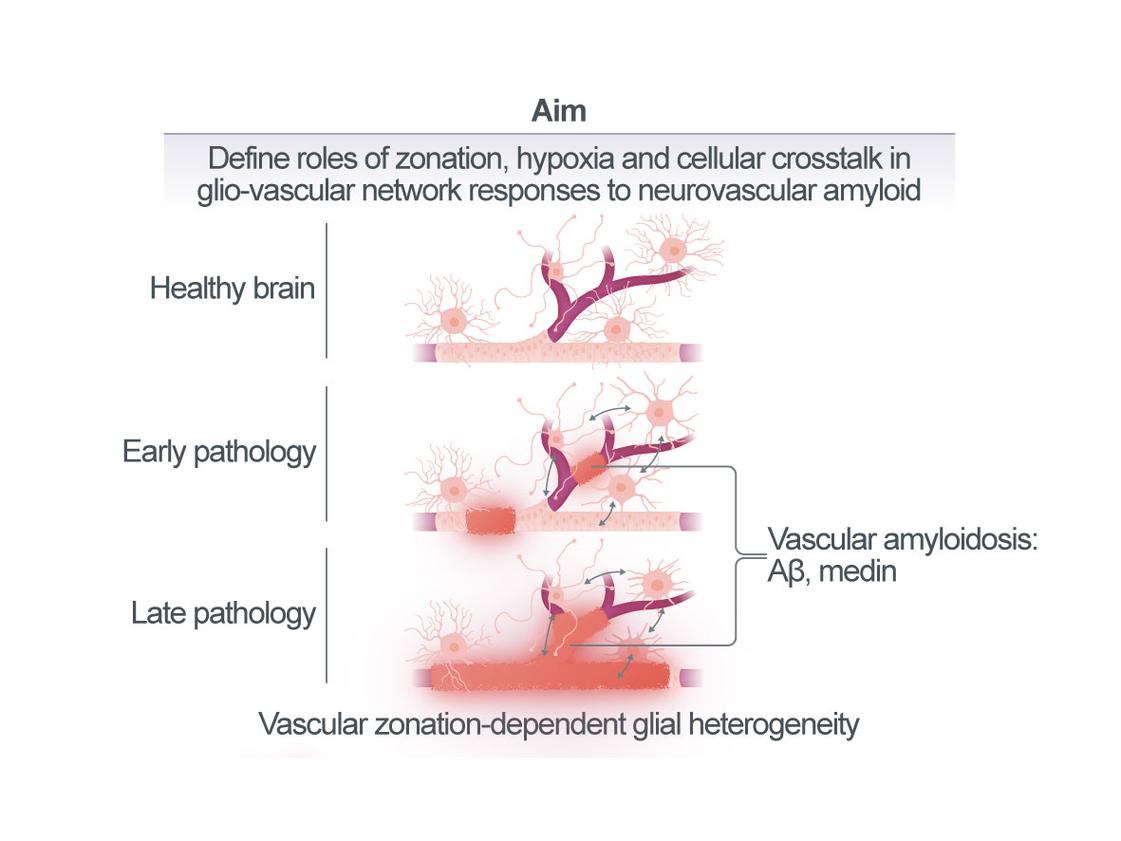
Defining the interplay of vascular and glial cellular networks in response to neurovascular amyloidosis
Age- and disease-associated neurovascular amyloids, such as amyloid-β (Aβ) in patients with cerebral Aβ angiopathy (CAA) but also medin amyloid, disrupt vascular function and integrity in the brain, e.g., by causing arterial stiffening and hemorrhages, respectively. Blood protein extravasation due to micro-hemorrhages has been shown to trigger inflammatory microglial and astrocyte activation that may contribute to neuronal damage. However, it remains less clear whether already at earlier stages of vascular amyloid pathology, i.e., while the blood-brain barrier (BBB) is still intact, glia may be activated by brain hypoperfusion and hypoxia resulting from neurovascular dysfunction per se or through signals from vascular cells directly affected by amyloids. Such glial activation states are of high therapeutic interest, as they may be precursors to damaging cellular phenotypes that develop with progressive pathology. Therefore, this project will delineate the spatio-temporal relationship of neurovascular amyloid formation, vascular dysfunction, and the molecular responses of vascular and glial cell networks in our novel mouse models of human medin amyloidosis as well as an established mouse model of cerebral Aβ angiopathy (CAA), the APPDutch line. Across disease phases and vascular zones, we will i) perform in-depth histological analyses in mouse and human tissue (Aim 1) and ii) molecular profiling (Aim 2) as well as iii) functional in vivo imaging (Aim 3). Findings from these analyses will be validated in human tissue and iPSC-based models. Mechanistically, we will test if hypoxia-induced signaling drives microglial and/or astrocyte activation in vivo and in iPS cell systems and examine the consequences for pathological burden (Aim 2).
Thus, this project aims to determine how disruption of the vascular cell network by local amyloid deposition and the resulting molecular responses in the glial compartment may eventually evolve to generate an inflammatory state that is detrimental to brain health. We expect the results to provide novel insights into key aspects of age- and disease-associated neurovascular amyloid pathogenesis, ultimately accelerating the translation of research findings into clinical applications, including the identification of biomarkers and the development of new therapeutic approaches.