Research Area A: Vascular Compartments
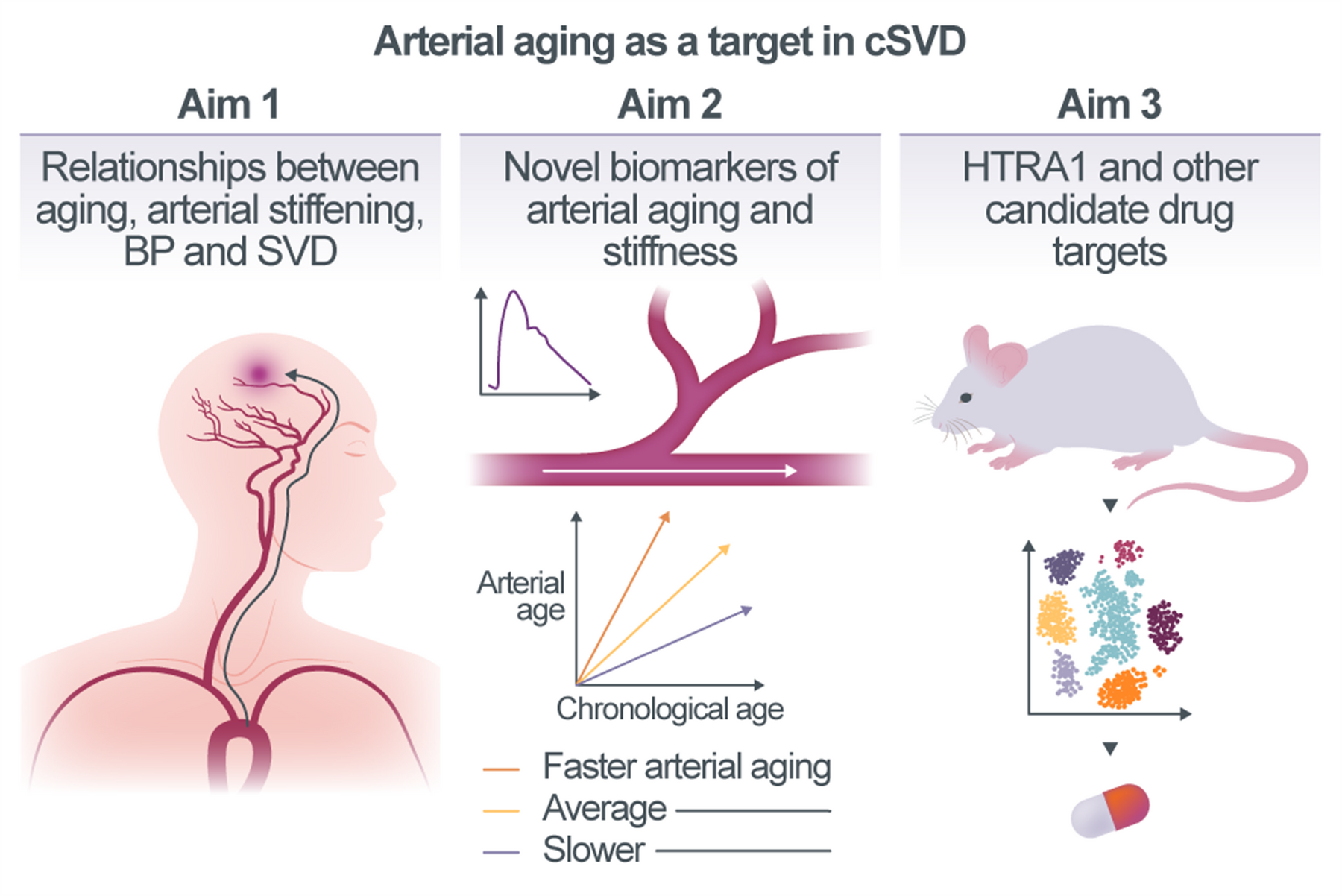
Arterial aging as a target for lowering the burden of cerebral small vessel disease
Cerebral small vessel disease (cSVD) is a major cause of stroke and dementia in the elderly population. Aging, the primary risk factor for SVD, results in stiffening of large arteries reducing their hemodynamic buffering capacity and allowing pulsatile blood flow to propagate to the downstream vasculature. This can cause damage to small blood vessels of highly perfused organs, such as the brain, which could provide an explanation for the relationship between aging and pathologies across different vascular segments. The overarching objective of this project is to investigate zonation-dependent molecular, cellular, and physiological effects of arterial aging as a cause of SVD. Specifically, we will integrate large-scale population-based multi-omics and imaging data, as well as data from genetic animal models of SVD in order to: (i) dissect the relationships between aging, arterial stiffening, blood pressure, and risk of SVD in humans (Aim 1), (ii) identify quantifiable biomarkers of arterial aging (Aim 2) and, (iii) profile proteomic, transcriptomic, ultrastructural and functional changes across the arterial tree during physiological mouse aging and in Htra1-related SVD (Aim 3).
Project A02 | Martin Dichgans
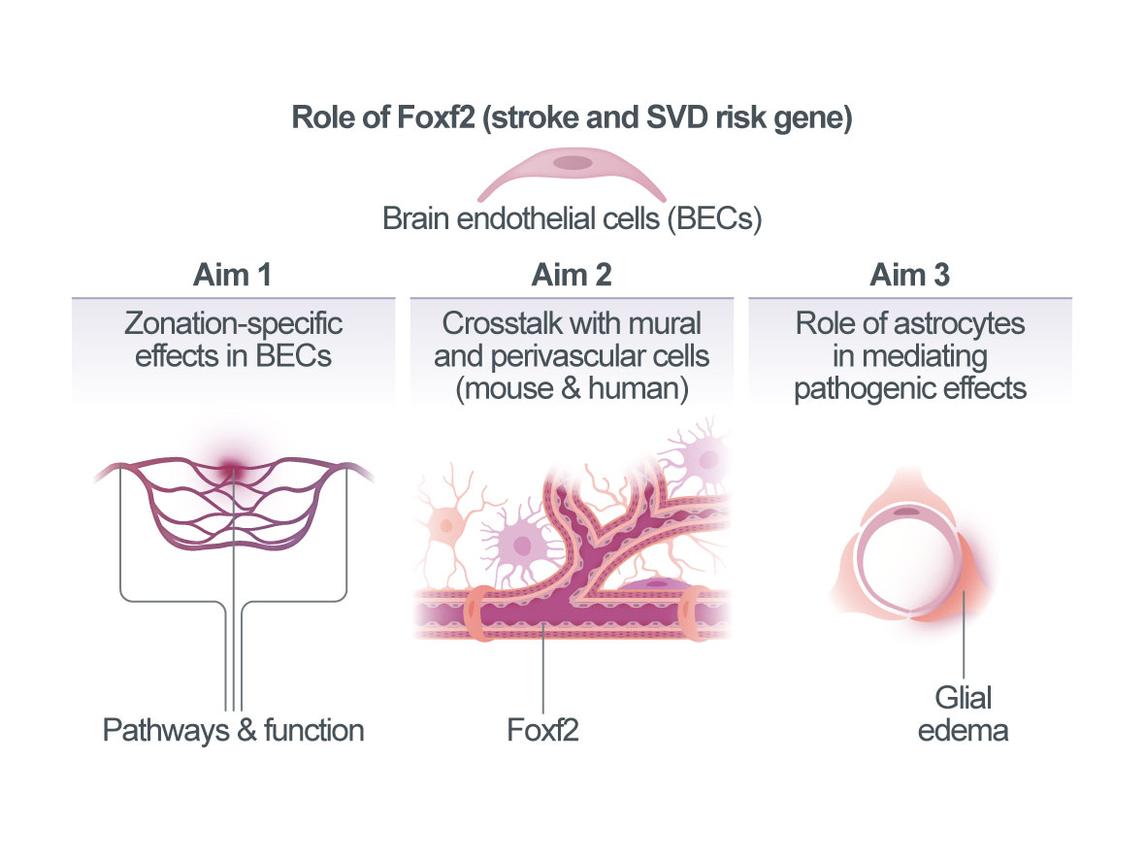
Zonation-specific mechanisms in Foxf2-related small vessel disease
Cerebral small vessel disease (SVD) accounts for most hemorrhagic strokes, 25% of ischemic strokes, and contributes to at least 40% of dementias. However, its mechanisms are poorly understood and in contrast to atherosclerotic disease there are no proven treatments for SVD. Recent results from clinical studies, human genetics, and experimental models point to a role of endothelial dysfunction in SVD. Brain endothelial cells (BECs) have unique functions at the blood-brain barrier (BBB) and in controlling cerebral blood flow (CBF). They are a focal point for vascular signaling, and for integrating cellular, blood-borne, and mechanical stimuli. Acutely, this input is translated into electrical or molecular signals that influence resting CBF, while redirecting local flow to regions of increased cellular activity. BECs further engage in sustained crosstalk with mural cells (smooth muscle cells [SMCs], pericytes), as well as astrocytes, potentially mediating BEC-related effects in SVD. We recently identified FOXF2 (Forkhead Box F2) as a major risk gene for stroke and SVD through genome-wide association studies (GWAS). Common variants at FOXF2 associate with small vessel stroke and neuroimaging markers of SVD in humans. The risk allele lowers FOXF2 expression levels, suggesting a requirement of Foxf2 for proper vascular function and brain homeostasis. Indeed, inactivation of Foxf2 in adult mice causes astrocytic endfeed swelling and neuronal injury. Importantly, expression of Foxf2 in the brain is largely restricted to endothelial cells (ECs) and pericytes10. Using a new mouse line with inducible inactivation of Foxf2 ECs (Cdh5-CreERT2;Foxf2fl/fl or “Foxf2iECKO”) we recently demonstrated a requirement of endothelial Foxf2 for maintaining vascular function (BBB integrity and functional hyperemia). We further showed that endothelial Foxf2 deficiency exacerbates infarct size in experimental stroke. However, the precise location of BEC dysfunction with respect to vascular zonation (arteriole - capillary - venule) and the ensuing molecular, cellular, and functional effects on mural cells, astrocytes, microglia, and other cell types remain unknown. The overarching goal of this project is to uncover vascular zonation-specific mechanisms of Foxf2 and their role in SVD and stroke. We will i) determine zonation-specific effects of endothelial Foxf2 on vascular function and experimental stroke and relate these effects to zonation-dependent molecular pathways; ii) study the crosstalk between BECs and other vascular cell types accounting for vascular zonation; and based on our finding of astrocytic endfeed swelling in Foxf2iECKO mice, we will iii) test the hypothesis that astrocytes mediate effects of endothelial Foxf2-deficiency on relevant outcomes (vascular function, neuronal integrity, experimental infarcts, behavior). We expect the results to provide fundamental insights on neurovascular physiology in SVD and stroke and open avenues for BEC-based therapeutic approaches.

Investigating the role of FOXF2 in regulating the neurovascular compartment using a human iPSC-based 3D BBB model.
Cerebral Small Vessel Disease (SVD) is a significant contributor to stroke and dementia yet lacks proven treatments. SVD has a strong genetic component and FOXF2 was recently identified as a major risk gene for stroke and SVD. Our previous research indicates that loss of FOXF2 function affects the cellular network within the neurovascular compartment at multiple levels, but the detailed mechanisms still require further investigation. So far, SVD has mostly been studied in animal models, which - while physiologically relevant - have limitations due to underlying genetic, molecular, and immunologic differences between the human and animal blood-brain barrier (BBB). These differences hinder the effective translation of findings to human patients, as evidenced by high failure rates (≥80%) in clinical trials of drugs validated in animals. Human models based on primary cells or induced pluripotent stem cells (iPSCs) have been developed to fill this gap, but most available co-culture and transwell models represent an oversimplification of the BBB and do not faithfully recapitulate the human situation, which makes disease modeling challenging. This project focusses on applying a novel iPSC-based human 3D model of the neurovascular compartment, which due to its fully iPSC-derived origin and faithful recapitulation of central BBB features overcomes these drawbacks and allows to mechanistically investigate how loss of FOXF2 contributes to SVD. Aim 1 will focus on further optimizing the already established FOXF2KO 3D BBB model and developing assays to characterize disease-relevant roles of cellular crosstalk between different BBB cells, perfusion and shear stress, as well as vascular zonation; in Aim 2 we will investigate how neurovascular dysfunction due to FOXF2 deficiency affects surrounding human neurons by altered iron transport, which emerged as a potentially disease-relevant pathway in preliminary mouse experiments; and in Aim 3 we will explore consequences of FOXF2 deficiency on BBB-specific lipid composition, a critical component of BBB formation that may be dysregulated together with other BBB hallmarks upon loss of FOXF2KO. Our project aims to not only provide a human iPSC-based model of the neurovascular compartment revealing novel disease mechanisms in SVD but also help identifying novel targets for interventions and provide opportunities to test these directly in a powerful human system in vitro. A successful application of our new model for investigating mechanisms of FOXF2 deficiency in SVD will also validate its applicability for investigating other genetic risk factors of neurovascular diseases (NVD) in future research.
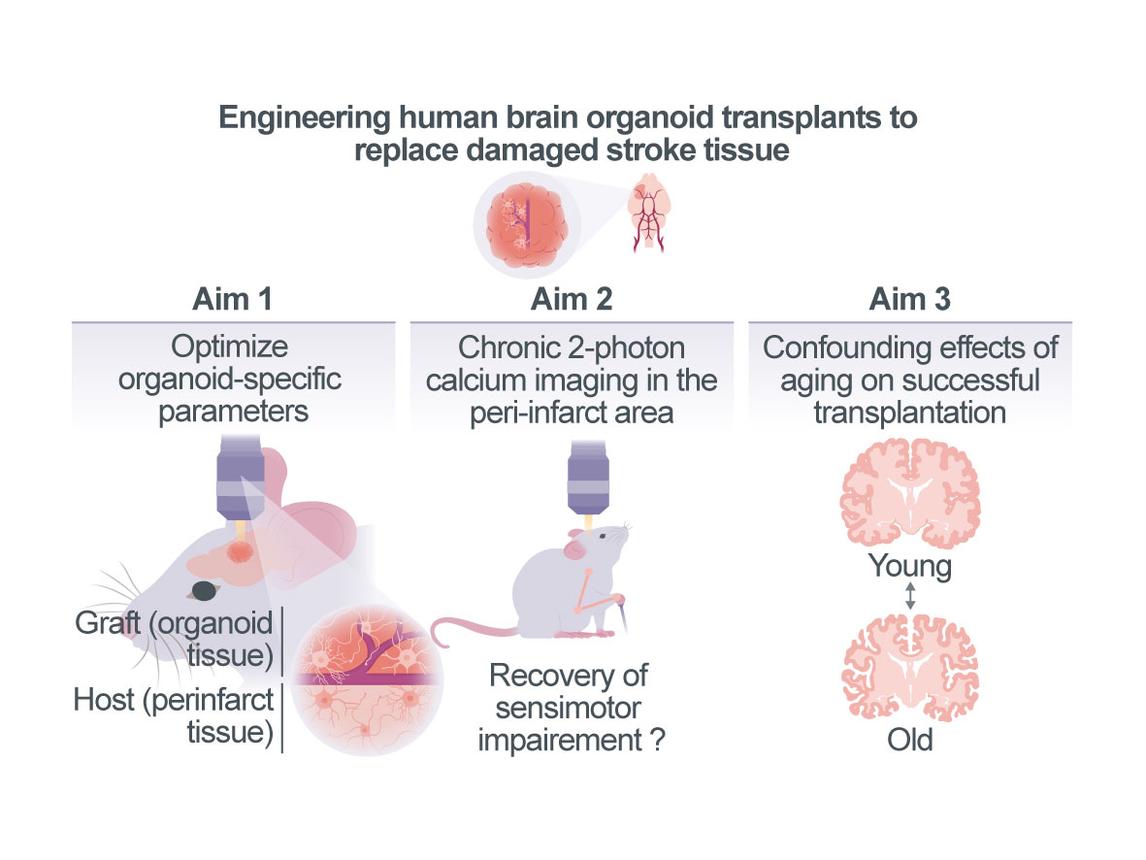
Probing tissue replacement strategies as a therapeutic intervention to facilitate restoration of the peri-infarct region after stroke
Large infarcts induce vast structural damage in the brain, limiting spontaneous recovery and functional compensation. Considering the lack of specific treatment strategies, personalized human stem cell-based tissue engineering technologies might offer a promising avenue for therapeutic interventions. However, a prerequisite for the successful transplantation of neuronal tissue is its connection to the vascular supply system and its integration into the neuronal network of the surviving, peri-infarct area. The Schäfer lab recently developed an approach that allows transplanting human stem cell-derived cortical brain organoid tissue into mice, enabling the tissue to receive supply from the mouse vascular system1. Here, we propose to combine the expertise of the Schäfer laboratory with that of the Wahl group, who recently established a stroke mouse model, where chronic live 2-photon calcium imaging of neurons can be performed in the peri-infarct area before and after a unilateral photothrombotic stroke affecting large sensorimotor areas. Our overarching goal is to probe a novel organoid-based tissue replacement strategy for its ability to restore the perturbed multi-cellular interactions of the peri-infarct region, thereby inducing recovery of lost sensorimotor function. To achieve this goal, we will in Aim 1 first optimize organoid-specific parameters (e.g. composition of neuronal and glial cells in the organoid) that facilitate successful integration into the peri-infarct tissue. In Aim 2 we will perform chronic 2-photon calcium imaging in the peri-infarct area and the organoid before and after stroke while animals perform a grasping task, which will allow us to assess whether recovery of lost grasping function can be promoted by this cell replacement therapy. We will apply generalized linear models to predict sensorimotor outcome depending on vascular parameters, neuronal activity and neuron-glia interactions. In Aim 3 we will use aged mice (>12 months old) to study the confounding effects of aging, which might influence the efficacy of successful transplantation as well as the level of functional recovery achievable. Examining how the transplanted tissue integrates in the surviving, functional networks of the peri-infarct region could provide novel avenues for personalized cell transplantation and replacement strategies for stroke patients suffering from chronic motor impairment.
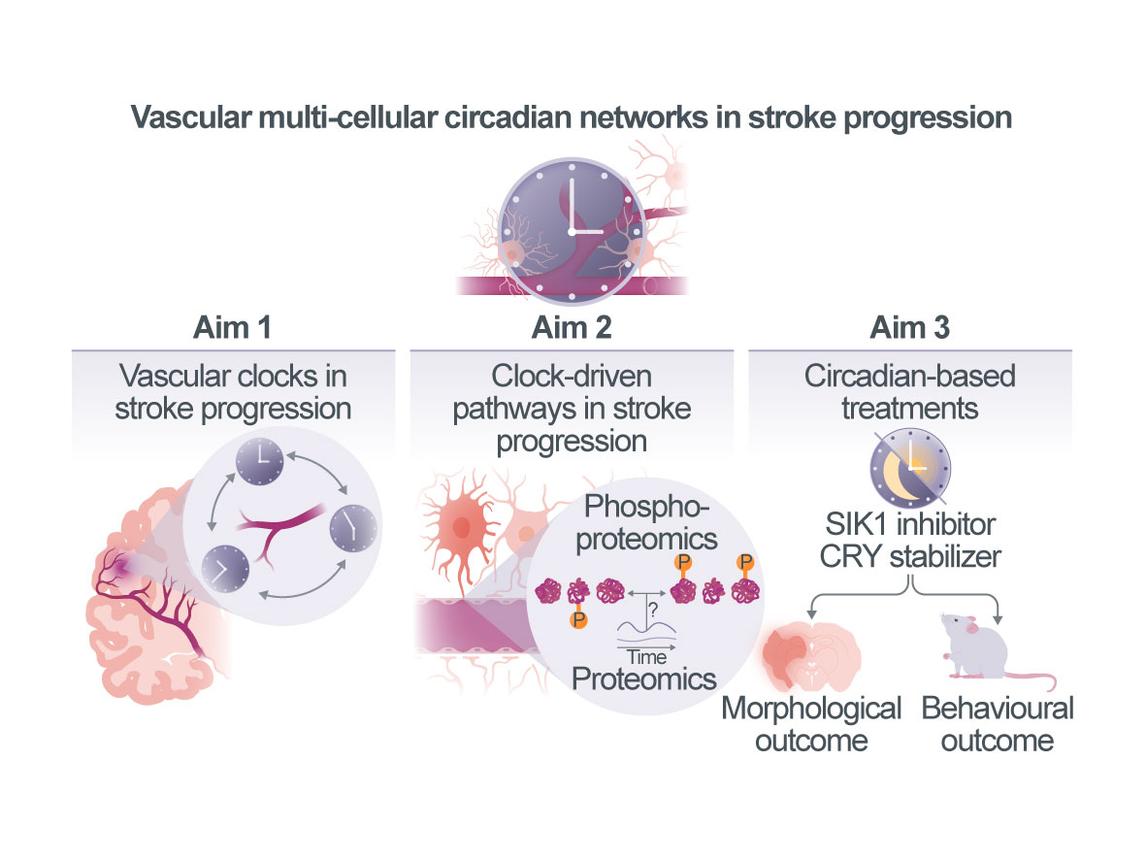
Vascular multi-cellular circadian networks as a modulator of stroke progression
Ischemic stroke remains a leading cause of death and disability. A major unresolved challenge is understanding how infarcts evolve from the initial ischemic core to the final infarct. Emerging clinical and experimental evidence suggests that circadian clocks – the intrinsic 24-hour timing systems present in nearly all cells – play a crucial role in modulating stroke outcomes. Circadian rhythms are governed by interlinked transcriptional, translational, and post-translational feedback loops, regulating approximately 25% of gene expression, 10% of protein abundance, and 30% of post-translational modifications. In the brain, these rhythms orchestrate key physiological processes such as sleep, vascular tone, blood–brain barrier (BBB) function, and cell death. Notably, recent studies have demonstrated that the time of stroke onset significantly influences collateral flow and infarct progression with strokes occurring during the inactive (sleep) phase leading to poorer outcomes – independent of time to treatment. Our preliminary work in mice further supports this concept. We show that exposure to white light at night (WLAN) – corresponding to the mice’s active phase – alters clock gene expression in neurons and vascular cells and exacerbates infarct sizes. These effects are mitigated in mice with targeted deletion of core clock genes (e.g., Cryptochrome [CRY] 1/2, Bmal1) suggesting that the detrimental effect of WLAN is clock-dependent. These findings underscore a critical, yet underexplored, role of circadian biology in stroke evolution and raise the possibility that targeting circadian mechanisms may open new therapeutic avenues to improve outcomes after stroke. The overarching goal of our project is to elucidate how circadian clocks within neurovascular multicellular networks – specifically the interplay among endothelial, mural, and neuronal clocks – modulate stroke progression and outcomes. To this end, we will employ genetic tools, advanced imaging, and multi-omics to: (i) characterize neurovascular circadian clock function at high cellular, spatial (vascular zonation-specific), and temporal resolution during stroke evolution, and determine how post-stroke sleep disruption impairs clock activity; ii) dissect the role of neurovascular multi-cellular circadian networks in regulating blood–brain barrier permeability and vascular tone during stroke; and iii) determine whether restoring or strengthening vascular clock function improves stroke outcomes, thereby paving the way for clinical translation.
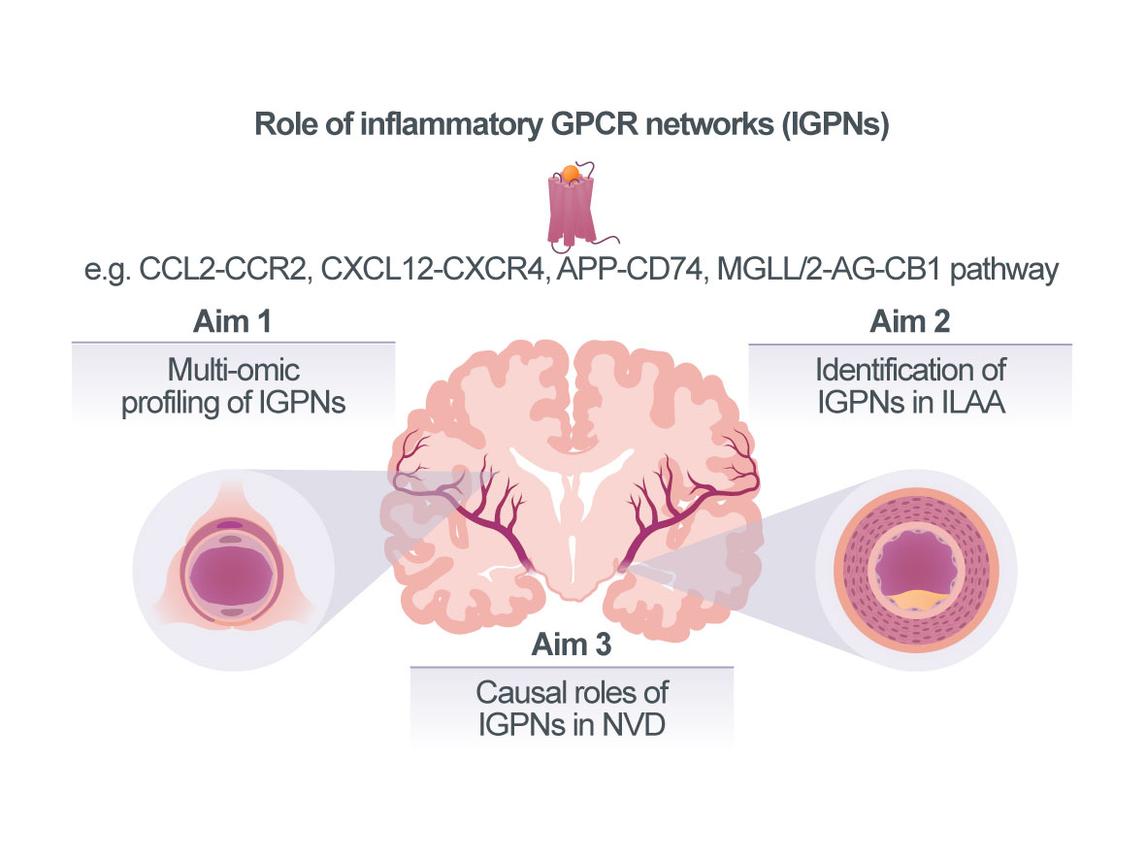
Compartmentalized diversity of inflammatory GPCR networks in neurovascular disease
Vascular inflammation is a major cause of cardiovascular diseases (CVDs). The underlying mecha-nisms have been extensively studied in arterial vascular segments associated with extracranial atherosclerosis. In fact, an increasing number of clinical studies provides proof for the therapeutic utility of anti-inflammatory approaches in atherosclerotic CVDs, but current drug strategies still lack specificity for vascular targets and suffer from immuno¬suppressive side effects. Neurovascular diseases (NVDs) including cerebral small vessel disease (cSVD) and intracranial large-artery atherosclerosis (ILAA) account for a large fraction of strokes and dementias. However, while it is established that the affected vascular segments in extra- vs intracranial vascular disease differ in size and anatomy and despite initial correlative evidence linking inflammation and modifiable cardiovascular risk factors such as obesity, type-2 diabetes (T2D), and hypertension with NVD, the extent and mecha¬nistic understanding of how vascular inflammation drives NVDs is incomplete. Accordingly, treatment options for cSVD and ILAA are currently limited to antithrombotics, antihypertensives, and lipid-reducing drugs, whereas vascular-targeted anti-inflam¬ma¬tory therapies have yet to be established. To this end, chemokines and certain lipid mediators that signal through G protein-coupled receptors (GPCRs), constituting Inflammatory GPCR Net¬works (IGPNs), represent promising next-generation vascular targets with therapeutic promise. We have extensively studied the atypical chemokine (ACK) MIF, its paralog MIF-2/D-DT, their receptors and the ACK interactome, as well as cannabinoid (lipid)-sensi¬tive receptor networks in athero¬scle¬rosis. These and other studies indicate that IGPNs have key pathogenic roles in atherosclerosis of extra¬cranial arteries, but their contribution to cSVD and ILAA is poorly understood. Further-more, the distri¬bution of IGPNs along the vascular segments associated with NVD is un¬known. We analyzed single-cell-RNAseq data sets from cerebral vasculature and identified cell-specific expression patterns and disease associations of IGPNs, and obtained initial evidence for contributions to cell-communication pathways. Together with preliminary qPCR data from different vascular beds and a receptor knockout (KO) mouse model, this lends support for a compartmentalized role of IGPNs in NVDs.
Our overarching goal is thus to comprehensively profile IGPNs in NVDs and identify candidate IGPN components as potential future therapeutic targets. Capitalizing on tissue, mouse and cell model resources in the CRC, we will first profile IGPNs in cSVD and study the regionality of candidate chemokines, lipid mediators, and their receptors (Aim 1). Furthermore, we will establish experimental in vivo and in vitro models of ILAA and obtain human ILAA biobank specimens to extend IGPN profiling to another neurovascular condition and define IGPN differences between extra- vs intracranial atherosclerotic vascular segments (Aim 2). Pre-identified targets such as CXCL12/CXCR4, MIF/CD74, or the MGLL/2-AG/CB1 axis will be studied for causality by hypo-thesis-driven pharmacological blocking approaches (Aim 3), which will be extended to selected segment-specific chemokines or lipid agonists identified in Aims 1/2. We expect to obtain novel information on IGPNs in cSVD, examine their patterns in different arterial regions to understand their role in ILAA, and explore therapeutic strategies based on identified causal IGPN pathways.
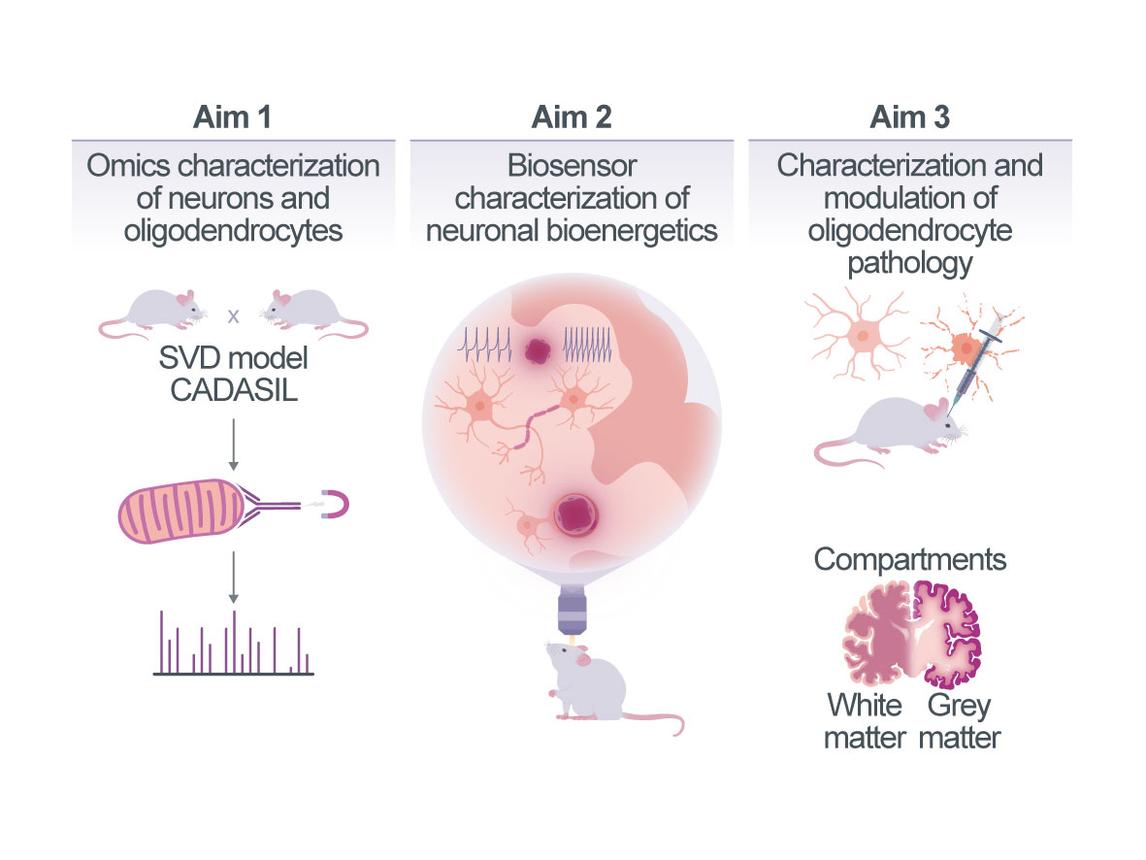
Bioenergetic failure of neuro-glial cell networks in small vessel pathology
As small vessel diseases (SVDs) progress, neural networks malfunction and can finally degenerate1. SVD is accompanied by increased oxygen extraction rates as an indication of metabolic stress and of mismatch between bioenergetic substrate (e.g., oxygen and glucose) supply and demand2. However, the pathophysiological link between primary small vessel pathology and neuronal dysfunction remains incompletely understood3. Here, we will pursue the hypothesis that small vessel pathology results in a progressive restriction of bioenergetic substrate supply, creating critical gradients and undernourished “zones” within neuropil relatively distant from capillaries, which cannot fully support the local demand of the most energy-dependent cell types. We expect that this will lead to local decompensation and dysfunction, especially of energetically susceptible fast-firing myelinated axons. We anticipate this pathology to start remote from capillaries, initially creating an “outside-in” pattern of pathology. Based on this hypothesis, in Aim 1, we will probe the pattern of SVD pathology in a mouse model of CADASIL (cerebral autosomal-dominant arteriopathy with subcortical infarcts and leukoencephalopathy) caused by Notch3 mutations. We will analyze the spatial relationships between vessel proximity and early signs of neuro-glial disruptions, comparing gray and white matter, establish the dynamics of capillary changes by in vivo imaging in CADASIL mice, and further examine autopsy samples from patients with CADASIL and sporadic SVD. Aim 2 will characterize the bioenergetic – and especially mitochondrial – adaptations of susceptible neurons and their projections in relation to capillary proximity and vessel remodeling. Such adaptations can involve structural, functional, and molecular changes that adapt a cell’s metabolism to changing bioenergetic supply and will be characterized using in situ phenotyping of mitochondria guided by cell-type-specific transcriptomics/proteomics and complemented with biosensor ATP imaging. We will also induce capillary remodeling, using two-photon laser-induced small vessel occlusions (optoSVOs) as a comparison to spontaneous remodeling in SVD and as a secondary challenge. Guided by our molecular and functional phenotyping, we will modulate bioenergetic resilience using viral gene modifications to probe the effects on axon-glial integrity. Finally, in Aim 3 – as myelination is an important transcellular metabolic and bioenergetic adaptation of axons that is susceptible to SVD –, we will study oligodendrocyte (OL) and myelin pathology in CADASIL mice during SVD progression and in response to optoSVOs (alone or as a secondary challenge in CADASIL mice). For this, we will image OLs, the axons they sheath, and nearby capillaries longitudinally and characterize the bioenergetic state of OLs (ATP biosensor imaging, cell-type-specific omics). Based on the results, we will use pro-myelinating interventions to study their efficacy in SVD and understand the potential for and effects of protecting myelin on axonal vulnerability.