Research Area B: Immune Compartments
Vascular zonation-specific mechanisms of the post-stroke vascular-microglia interaction
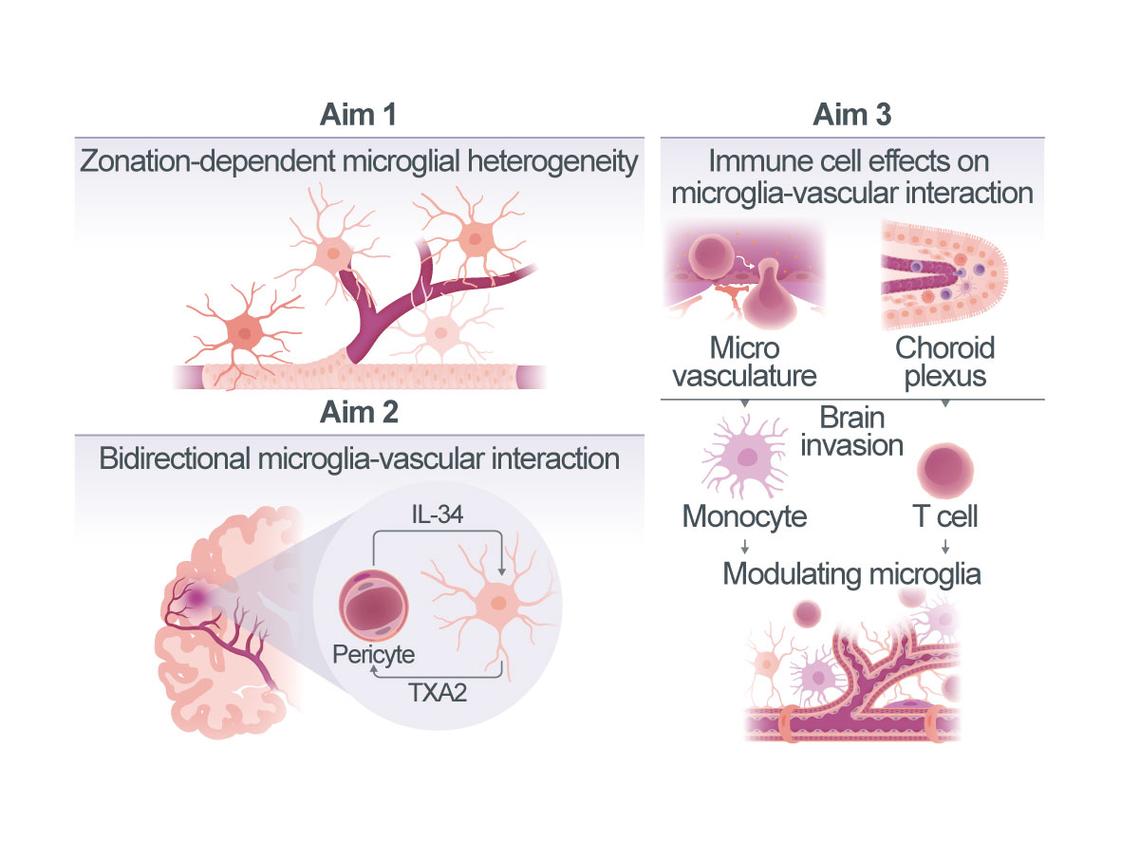
Ischemic stroke initiates a complex cascade of immune and vascular responses that critically shape long-term outcome. Among immune cells, microglia are increasingly recognized not only as inflammatory regulators, but also as modulators of cerebral blood flow and vascular homeostasis. Recent work, including our own, suggests that microglia interact with vascular cells in highly structured, compartmentalized ways, influencing local perfusion through physical contacts and the release of vasoactive factors. However, the molecular mechanisms underlying this bi-directional communication and its spatial organization along the vascular arborization remain unclear.
Our preliminary data reveal a novel functional axis between microglia and pericytes in the post-stroke brain: microglia release thromboxane A2 (TXA2), a potent vasoconstrictor that we found to act on Thromboxane receptors selectively expressed in the brain by pericytes, while pericytes in turn produce IL-34, a trophic factor acting via microglial CSF1R to modulate microglial reactive cell states. These interactions suggest the existence of a dynamic feedback loop that controls local vascular tone and neurovascular coupling. Notably, both TXA2 and IL-34 appear to operate in a zonation-specific fashion, confined to the microvascular level.
Based on these novel findings, this project aims to dissect the molecular mechanisms of microglia-vascular interactions in stroke recovery. We will focus on (1) defining how microglia modulate cerebral perfusion in a vascular zonation-specific manner, (2) elucidating the underlying signaling pathways of microglia-pericyte crosstalk and its role in regulating vascular tone, and (3) investigating how peripheral immune cell infiltration in the context of post-stroke neuroinflammation alters the microglial influence on the vasculature. To address these questions, we will combine spatial transcriptomics, in vivo widefield imaging, and behavioral analysis in mouse models with conditionally-altered TXA2 and IL-34 signaling. By resolving how microglia integrate into compartmentalized vascular networks and shape cerebral perfusion, this work will provide novel insight into multicellular immune-vascular communication in neurovascular diseases. The findings are expected to identify new molecular targets for promoting functional recovery after stroke and contribute to the CRC1744’s overarching aim of decoding spatially organized cellular networks in NVDs.
Deciphering the role of perivascular inflammation after subarachnoid haemorrhage
Subarachnoid haemorrhage (SAH) is a severe subtype of stroke caused mainly by the rupture of an intracranial aneurysm. Patients who survive the acute haemorrhage often experience delayed cerebral ischemia (DCI), which can lead to unfavourable long-term outcomes. DCI can occur already within the first few hours after SAH, but the exact pathomechanisms of DCI are unclear and therapeutic options remain mainly symptomatic and marginally effective.
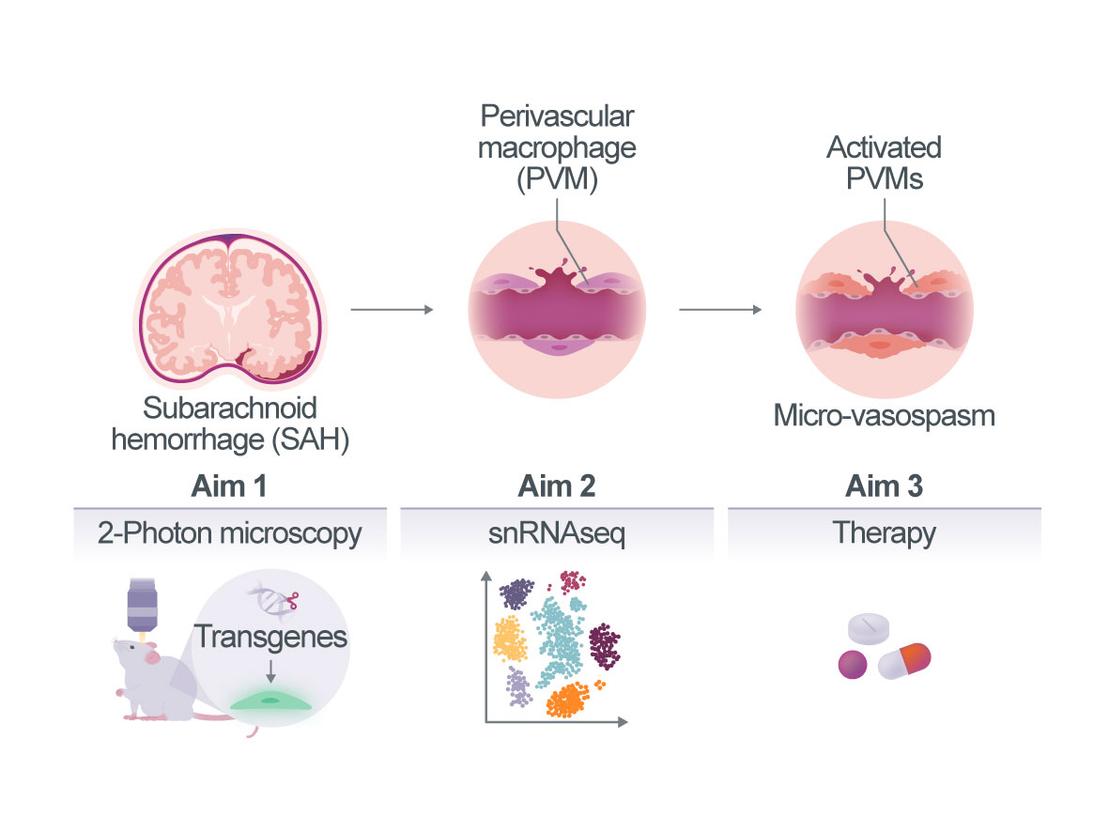
Using in vivo imaging in patients and experimental animals, we and others have shown in recent years that the cerebral microvascular dysfunction is a major cause of early DCI after SAH. Our most recent data suggest that perivascular macrophages (PVM) play a central role in this process. However, the cellular interactions and molecular mechanisms that ultimately link PVM to microvascular dysfunction after SAH are unknown.
Therefore, the main aim of the current project is to decipher the molecular mechanisms by which PVMs interact with the vascular wall (pericytes, endothelial and smooth muscle cells) and adjacent parenchymal cells (astrocytes and neurons) to cause microvasospasm and microvascular dysfunction after SAH (cellular zonation). Further, we will try to understand how blood in the subarachnoid space is able to induce dysfunction in specific segments of the cerebral vasculature, i.e. cerebral capillaries (vascular zonation). To this end, we will investigate the morphological and functional changes of PVM after SAH by longitudinal in vivo multiphoton microscopy (Aim 1) and identify the genes and pathways activated in PVM and their neighbouring cells (endothelium, mural cells, astrocytes, and microglia) by single nucleus RNA sequencing (Aim 2). Using this knowledge, we will validate novel therapeutic strategies for the treatment of microvascular dysfunction following haemorrhage (Aim 3).
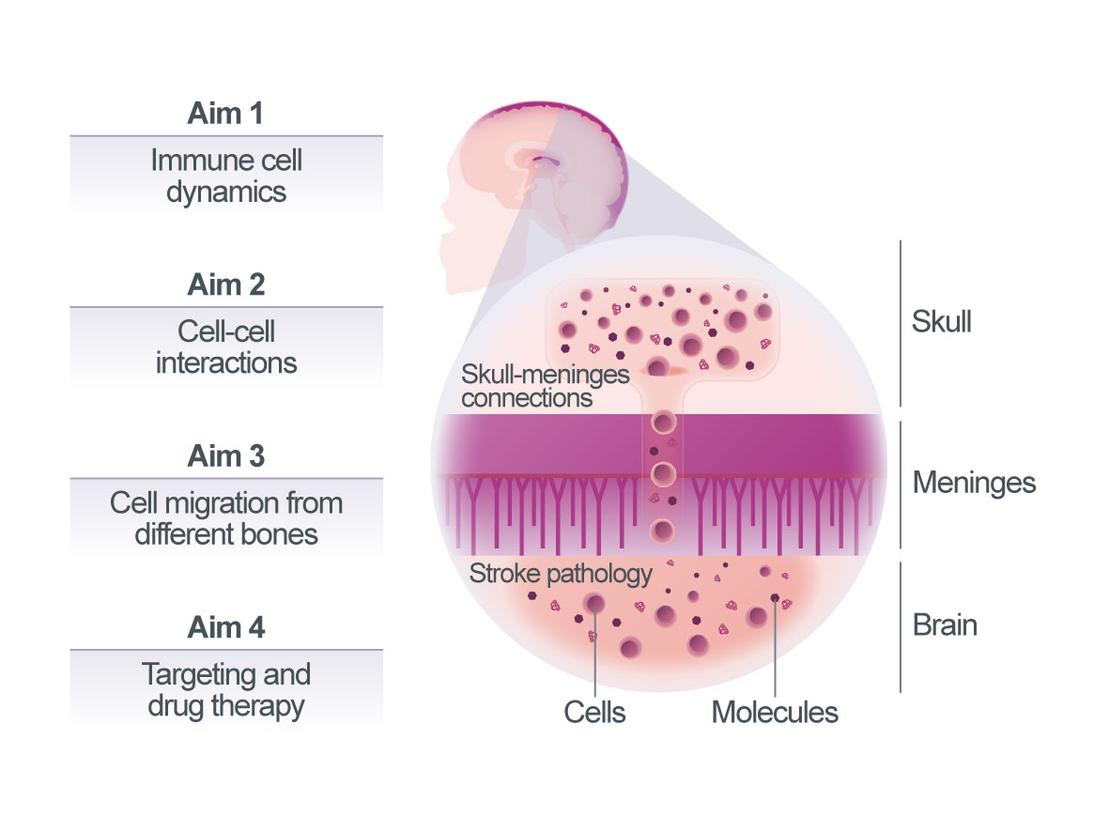
Skull-meninges connections in post-stroke neuroinflammation
We and others have recently discovered the close connections between the brain and the skull bone marrow through skull-meninges connections (SMCs). These SMCs are functionally important, as shown by a distinct expression profile of skull-resident immune cells. After the stroke, these cells show increased trafficking through SMCs, alongside activation. In patients, a skull-specific TSPO-PET signal correlates with disease severity across multiple neurological conditions1. To investigate the functional relevance of skull bone marrow for stroke outcome, we will pursue four aims:
(1) map the location of skull-derived immune cells post-stroke using DISCO tissue clearing and light-sheet microscopy. (2) map their interactions with meningeal cells using spatial transcriptomics and RABID-seq barcoded viral tracing. (3) compare migration patterns from skull, vertebrae, tibia, and femur marrow using differential labeling and adoptive transfer approaches; and (4) target key inflammatory regulators, including CXCR2, Nur77, and TREM1 pharmacologically (SB225002, Cytosporone B and TREM1 inhibitors) and genetically (e.g. conditional knockouts) to improve stroke outcomes.
Our transcriptomic profiling shows that Nur77 is significantly upregulated in skull marrow-derived neutrophils after stroke. Specifically, expression is 2.8-fold higher than in the tibia, suggesting a potential role in modulating their inflammatory behavior in this compartment. Although Nur77 is broadly expressed across immune and non-immune cells, its context-dependent regulation makes it an attractive candidate in the skull marrow niche.
We further build on prior studies showing that CXCR2 mediates neutrophil recruitment and influences infarct volume in cardiovascular injury models2, 3. We hypothesize that CXCR2-driven chemotaxis is functionally coupled to Nur77-mediated transcriptional control of neutrophil activity at the skull–meninges interface. TREM1, an amplifier of inflammatory signaling, also shows distinct regulation in the skull marrow after stroke. Its modulation may help balance beneficial and detrimental immune responses in this compartment. Together, these pathways represent a tightly regulated network controlling skull-derived neuroinflammation.
Our methodological pipeline will enable precise visualization and molecular characterization of this newly described neuroimmune pathway, laying the foundation for future therapeutic strategies targeting skull-specific neuroimmune pathways. We will define skull marrow–meningeal immune interactions as a novel regulatory axis in post-stroke inflammation, investigating key regulators including CXCR2, Nur77, and TREM1 to define new therapeutic strategies targeting skull-specific neuroimmune responses. In addition to these targets, transcriptomic analyses suggest that other immune regulatory molecules, such as CXCR4 and S100A8/9, may contribute to skull marrow-specific inflammatory reactions and will be further explored.
Molecular and cellular mechanisms of T cell infiltration and activation in the lesion after ischemic stroke
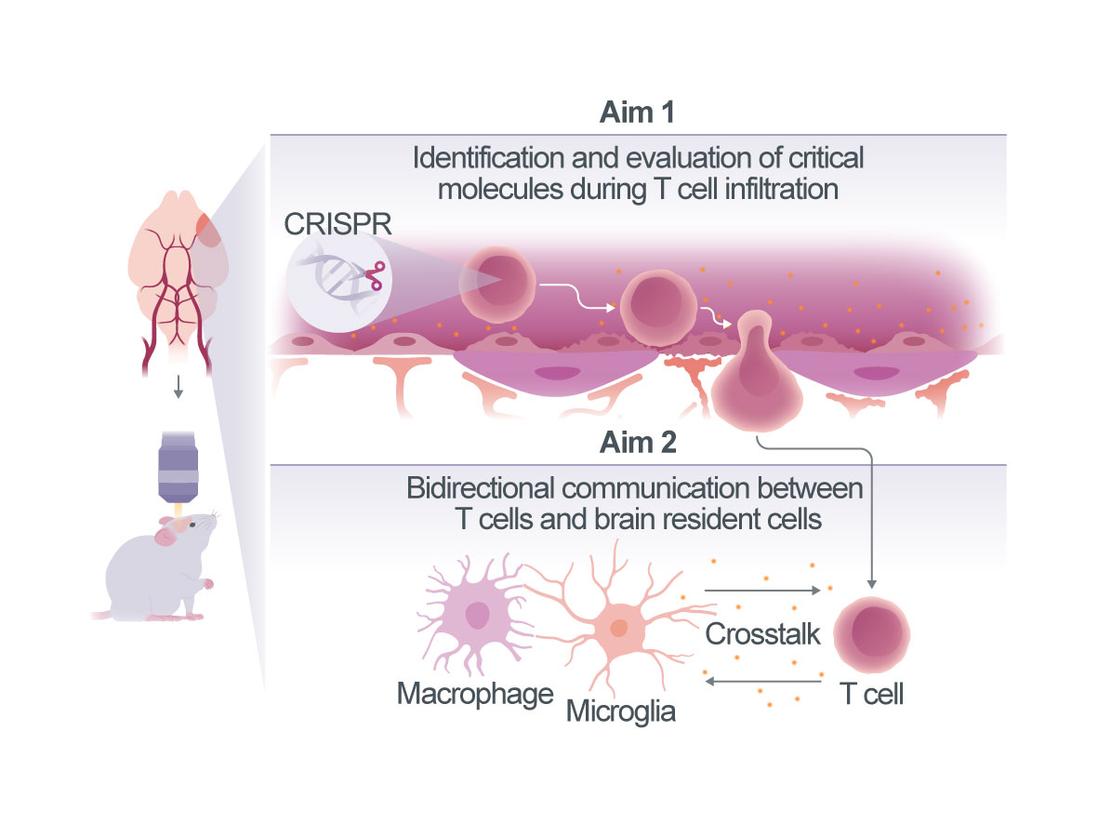
The pathophysiology of stroke is not solely determined by the initial ischemic insult but is significantly influenced by the subsequent immune response. Among the immune cells involved, CD4+ T cells are key mediators of secondary inflammatory responses1. Indeed, T cell repertoire includes several subsets with different functions in stroke. For instance, CD4+ T cells can differentiate into Th1, Th2, and regulatory T cells (Treg), each playing distinct roles in neuroinflammation2, 3. In addition, T cell subsets differentially affect the phenotype of their interacting partners including brain resident microglia4, 5. The diversity of T cell types and their interactions in different anatomical locations will be a key focus of this project, as these compartmentalized interactions contribute significantly to the progression of inflammation and tissue damage. However, the precise mechanisms governing the infiltration and interaction of CD4+ T cells within distinct locations: meninges (i.e. dura), choroid plexus and ischemic brain remain poorly understood.
In this proposal, we aim to elucidate fundamental questions: (1) How do CD4+ T cell subsets gain access to the brain following stroke? Which infiltration routes do they use (blood-brain barrier, choroid plexus and/or meninges), and are the invasion routes specific to T cell subsets (Th1 and Treg)? (2) How do brain-invading T cells interact with brain resident immune cells, including microglia, macrophages, and dendritic cells (DC) in specific brain compartments, including the meninges, parenchyma, and choroid plexus? What are the consequences of this interaction? Understanding these mechanisms are crucial for delineating the molecular and cellular pathways which drive T cell-mediated inflammation in stroke.
To achieve our research objectives, we will combine our expertise in intravital imaging, in vivo CRISPR-based gene editing, immune cell phenotyping, and T cell-microglia/macrophage/DC interaction studies in experimental stroke models4, 6-8. By manipulating gene expression in T cell subsets and visualization of their behavior in real-time, we aim to bring novel insights into the mechanisms of T cell entry and interaction with resident immune cells in the post-stroke brain. Ultimately, offering a unique perspective on their interactions with other cellular components, and potentially inform the design of innovative therapeutic strategies aimed at mitigating excessive neuroinflammation and improving stroke recovery.
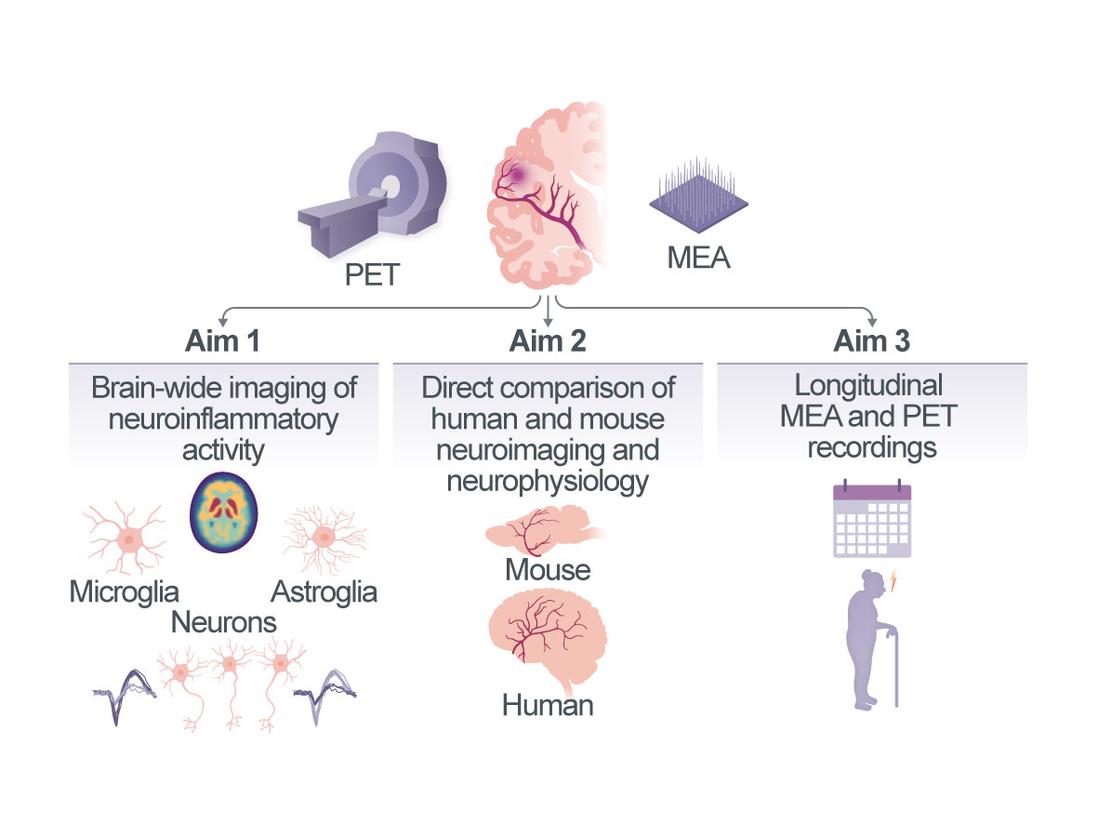
Interactions between stroke-induced neuroinflammatory responses and human brain reorganization at single-neuron resolution
Neurovascular diseases (NVDs) including stroke are a leading cause of death and disability. The reorganization of neuronal networks and thus the extent of recovery from stroke are governed by complex interactions between neurons and multiple other cell types (vascular, immune and glial) in distinct cellular compartments of the central nervous system. Neuroinflammation with activation of astrocytes and microglia is heavily implicated in the pathophysiology of ischemic stroke and one of the key factors influencing functional outcome1, 2. However, significant medical and technical challenges limit the study of these processes in humans, leaving our understanding of how stroke-induced inflammation affects human neuronal activity and circuit function very limited. In this project, we will use neuronal recordings with cellular resolution directly from the human brain and molecular imaging of inflammatory activity to provide first-of-its-kind insights into the crosstalk between the human micro- and astroglial compartment and populations of individual neurons and their cortical networks in NVD. We will (i) combine large-scale electrophysiological recordings of single-neuron activity with structural and functional MRI and PET imaging of neuroinflammatory responses in patients with chronic ischemic stroke. We will explore associations between the levels of micro- and astroglial activation and single-neuron activity and neuronal network viability both in perilesional brain areas and in distant, anatomically connected brain areas; (ii) perform the same multimodal measurements in mice after experimental stroke, which will allow us to verify the measured PET signals using cell sorting and FACS after radiotracer injection, histology and immunohistochemistry; (iii) include longitudinal measurements of microglial, astroglial and neuronal activity in both humans and mice and link these with measures of functional recovery after stroke. We expect this project will shed new light onto the long-standing question of whether stroke-induced neuroinflammatory responses are protective or harmful for human brain reorganization.